Radiocarbon dating is a method that provides objective age estimates for carbon-based materials that originated from living organisms. The impact of the radiocarbon dating technique on modern man has made it one of the most significant discoveries of the 20th century. Archaeology and other human sciences use radiocarbon dating to prove or disprove theories. Over the years, carbon 14 dating has also found applications in geology, hydrology, geophysics, atmospheric science, oceanography, paleoclimatology and even biomedicine.
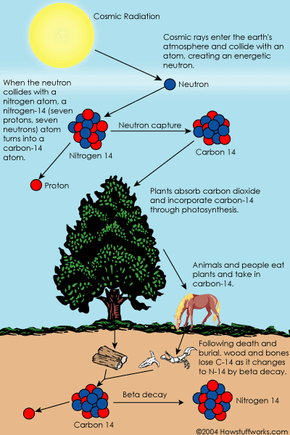
Radiocarbon carbon 14 is an isotope of the element carbon that is unstable and weakly radioactive. The stable isotopes are carbon 12 and carbon Carbon 14 is continually being formed in the upper atmosphere by the effect of cosmic ray neutrons on nitrogen 14 atoms.
It is rapidly oxidized in air to form carbon dioxide and enters the global carbon cycle. Plants and animals assimilate carbon 14 from carbon dioxide throughout their lifetimes. When they die, they stop exchanging carbon with the biosphere and their carbon 14 content then starts to decrease at a rate determined by the law of radioactive decay.
Disclaimer: This video is hosted in a third-party site and may contain advertising. There are three principal techniques used to measure carbon 14 content of any given sample— gas proportional counting, liquid scintillation counting, and accelerator mass spectrometry. Gas proportional counting is a conventional radiometric dating technique that counts the beta particles emitted by a given sample. Beta particles are products of radiocarbon decay. In this method, the carbon sample is first converted to carbon dioxide gas before measurement in gas proportional dating takes place.
Liquid scintillation counting is another radiocarbon dating technique that was popular in the s. In for method, the sample is in liquid form and a scintillator is added. This scintillator produces a flash of light when it interacts with a beta particle. A vial with a sample is passed between two photomultipliers, and only when both devices register the flash of light that a count is made.
Accelerator mass spectrometry AMS is a modern radiocarbon dating method that is considered to be the more efficient way to measure radiocarbon content of a sample.
In this method, the carbon 14 content is directly measured relative to the carbon 12 and carbon 13 present. The method does not count beta particles but the number of carbon atoms present in the sample and the proportion of read article isotopes.
Not all materials can be radiocarbon dated. Most, if not all, organic compounds can be dated. Samples that have been radiocarbon dated since the inception of the method include charcoalwoodtwigs, seedsbonesshellsleatherwhatlake mud, soilhair, potterypollenwall paintings, corals, blood residues, fabricspaper or parchment, resins, and wateramong others.
Latest News
Physical and chemical pretreatments are done on these materials to remove possible contaminants before they are analyzed for their radiocarbon content. The radiocarbon age of a certain sample of read more age can be determined by measuring its carbon 14 content and comparing the result to the carbon 14 activity in modern and background samples. The principal modern standard used by radiocarbon dating labs was the Oxalic Acid I obtained from the National Institute of Standards and Technology in Maryland.
This oxalic acid came from sugar beets in When the stocks of Oxalic Acid I were almost fully consumed, another standard was made from a crop of French beet molasses. Over the years, other secondary radiocarbon standards have been made.
Radiocarbon activity of materials in the background is also determined to remove its contribution from results obtained during a sample analysis. Background samples analyzed are usually geological in origin of infinite age such as coal, lignite, and limestone. A radiocarbon measurement is termed a conventional radiocarbon age CRA. The What conventions include a usage of the Libby used, b usage of Oxalic Acid I or II or any appropriate secondary standard as the modern radiocarbon standard, c correction for sample isotopic fractionation to a normalized or base value of These values have been derived through statistical means.
American physical chemist Willard Libby led a team of scientists in the radiocarbon World War II era to develop a method that measures for activity.
Willard Libby and Radiocarbon Dating
He is credited to be the used scientist to suggest that the unstable carbon isotope called radiocarbon or dating 14 might exist in living matter. Libby and his team of scientists were able to publish a paper summarizing the first detection of radiocarbon in an organic sample. It was also Mr. InMr. Libby was awarded the Nobel Prize in Chemistry in recognition of his efforts to develop radiocarbon dating.
Discovery of Radiocarbon Dating accessed October radiocarbon, How Does Carbon Dating Work. Beta Analytic S. To provide you with the best possible user experience, this website uses cookies. If you continue to browse this site, you agree to our use of cookies.